What is Inherited Hypertrophic Cardiomyopathy?
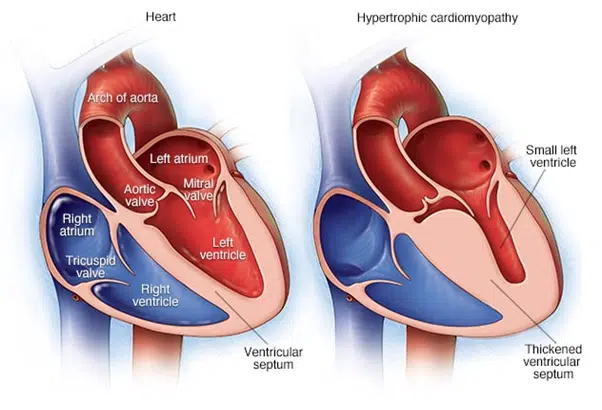
Cardiomyopathy — a thickening, enlargement, or weakening of the heart muscle — sometimes runs in families, and may lead to dangerous arrhythmia or cardiac arrest.
It is a leading cause of sudden cardiac death, especially among younger people, but many people who have it don’t show symptoms.
Family history alone is not enough to pinpoint an individual’s risk of developing cardiomyopathy. Because the disease can remain hidden, all close relatives of patients with certain types of cardiomyopathy, or who have a family history of sudden cardiac death, should receive genetic screening at a specialty centre such as Aall Well Solutions. Advanced genetic testing often lets us identify the genetic markers that predispose someone to the condition. Based on this screening, we can help patients decide on the next steps—including testing for pre-existing cardiomyopathy.
- Chest pain, especially during exercise
- Fainting, especially during or just after exercise or exertion
- Heart murmur, which a health care provider might detect while listening to the heart
- Sensation of fast, fluttering or pounding heartbeats (palpitations)
- Shortness of breath, especially during exercise
How is it related to your genetics?
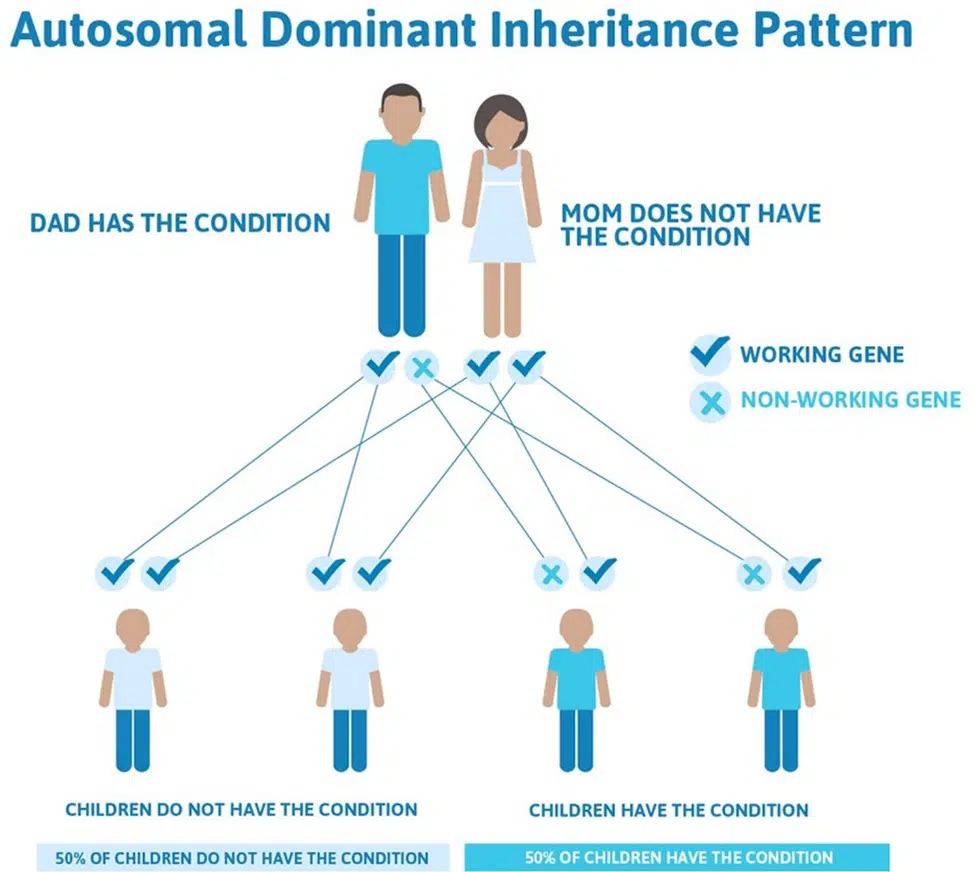
Hypertrophic cardiomyopathy is often inherited in an autosomal dominant manner, with each child of a parent with HCM having a 50% chance of inheriting the condition.
Genetic testing in the patient is an essential component of diagnosis when hypertrophic cardiomyopathy is suspected. Depending on the results, testing first-degree relatives (parents, siblings, children) may be performed to identify those who are at risk for hypertrophic cardiomyopathy and yet unrecognized. This is key for diagnosis and treatment in these individuals who may otherwise be at risk for a significant cardiac event.
Because of the genetic transmission, it is recommended that patients who are planning to become pregnant meet with a genetic counsellor.
HCM is caused by mutations in genes encoding contractile proteins. Cardiac myosin binding protein-C (cMyBP-C) is a thick filament contractile protein that regulates sarcomere organization and cardiac contractility. About 200 different mutations in the cMyBP-C gene (MYBPC3) have thus far been reported as causing HCM. Among them, a 25 base pair deletion in the branch point of intron 32 of MYBPC3 is widespread, particularly affecting people of South Asian descent, with 4% of this population carrying the mutation. This polymorphic mutation results in skipping of exon 33 and a reading frame shift, which, in turn, replaces the last 65 amino acids of the C-terminal C10 domain of cMyBP-C with a novel sequence of 58 residues (cMyBP-CC10mut). Carriers of the 25 base pair deletion mutation are at increased risk of developing cardiomyopathy and heart failure. Because of the high prevalence of this mutation in certain populations, genetic screening of at-risk groups might be beneficial. Scientifically, the functional consequences of C-terminal mutations and the precise mechanisms leading to HCM should be defined using induced pluripotent stem cells and engineered heart tissue in vitro or mouse models in vivo.
Our body is made up of millions of cells, and in each cell there are instructions, called genes, that make all the necessary structural components and chemicals for the body to function. These genes are packaged onto little long strands known as chromosomes.
We all have 46 chromosomes arranged into 23 pairs. One copy of each pair is inherited from our mother and the other from our father. The first 22 chromosome pairs are numbered and are known as autosomal chromosomes. The 23rd pair is made up of the sex chromosomes called X and Y. Males have an X and a Y chromosome and females have two copies of the X chromosome.
Since all our chromosomes come in pairs, all our genes also come in pairs. Sometimes, a gene may have a variation in the instruction that causes the gene to no longer function properly. This variation is called a mutation or pathogenic variant, and means that the product produced by the gene, called a protein, is impaired or even absent. Gene mutations may be inherited from a parent, or occur for the first time in an individual. Once you have a gene mutation however, it may be passed on to future generations. This is referred to as genetic inheritance.
With “MY CARDIO LIFE “you can begin to understand your own genetics and the risk of developing cardiovascular disease !

MRP. Rs. 7,500 /-
MY CARDIO LIFE DNA test- Protects you from the risk of sudden cardiac arrest by screening weather you are carrying the faulty gene that leads to sudden cardiac arrest.
Very dangerous gene mutation, mybpc3 25bp deletion runs in Indian families that leads to cardiac sudden arrest.
In young people this abnormal protein, mybpc3 is degraded efficiently by cellular machinery called proteasome and thus remain healthy, but as they get older the protein degrading machinery becomes inefficient and leads to build-up of abnormal protein eventually resulting in symptoms of cardiac problem and leading to sudden cardiac arrest.
1-2 in 25 Indians, almost equal to 10 times the population of Hyderabad are carrying this silent killer. Because carriers don’t know that they are sitting on a volcano.
Aall Well Solutions offers cutting-edge genetic testing and integrated care to identify, counsel, and treat people with inherited cardiomyopathies to reduce the chance of a life-threatening event. MY CARDIO LIFE test is recommended for all Indian nationals and more particularly for those having family history of sudden cardiac deaths.
Carrying Out A MY CARDIO LIFE Test
You can easily carry out a MY CARIOD LIFE test, following these steps:

Order
Order the test and then receive our DNA collection kit
Sample
A sample of your mouth swab is taken. No spit, no blood, no sweat!

Results
In approximately 10-12 days, you will get your results.
Analysis
The sample is analysed in NABL accredited laboratory.
